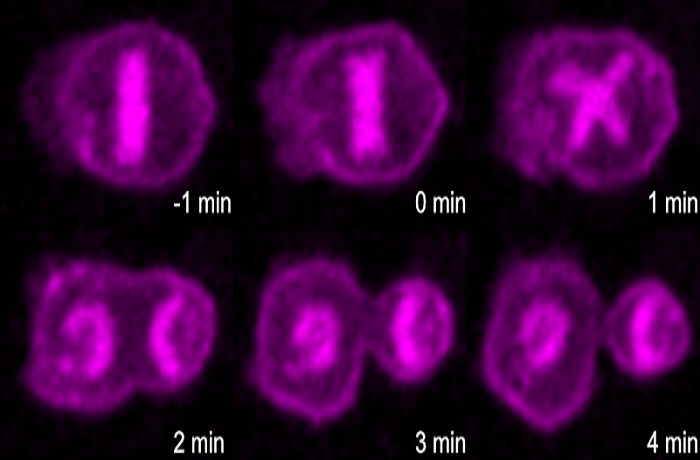
Apoptotic pathway promotes asymmetric cell division during C. elegans development.
Cell division doesn’t always produce identical daughter cells; often, the demands of multicellular development require cells to split into two quite different daughters with quite different fates. These “asymmetric” divisions are needed so that cells can differentiate and specialize, and some cells are even programmed to die shortly after their creation to ensure the proper function of the organism as a whole. In GENETICS, Mishra et al. found that the apoptotic cell death pathway regulates asymmetric division in the nematode worm Caenorhabditis elegans.
C. elegans is an exceptionally useful model organism for studying development because the fate of each of its relatively few cells can be precisely mapped. Many of the cells destined for death in the worm are actually the product of unequal division into a larger cell that differentiates and a smaller cell that undergoes apoptosis. The authors of the new report had previously studied the parent of one such uneven division, a cell known as the embryonic neurosecretory motor neuron neuroblast. They found that in the parental neuroblast, there is a gradient of activated CED-3 caspase, an executioner of apoptosis. This gradient leads to more active CED-3 caspase in the smaller daughter cell, which helps facilitate its death.
The authors wondered whether this CED-3 caspase gradient might be a general phenomenon in asymmetric divisions, so in the GENETICS report they studied another cell that divides into a large cell that survives and a smaller cell that dies: the QL.p neuroblast. The authors identified a similar CED-3 caspase gradient in these cells, showing that the phenomenon is indeed somewhat general.
Then, the authors used loss-of-function mutants to explore the role of the CED-3 caspase and its related pathways in the asymmetric division of QL.p. They found that disrupting the cell death pathway impaired the ability of QL.p to divide asymmetrically and could impact the fate of the daughter cells—often giving rise to two living cells, rather than one that lives and one that dies. Mutations in other genes associated with asymmetric division, like pig-1, also affected the fate of the daughter cells but did not change the CED-3 caspase gradient.
The authors explain that, in QL.p, two molecular gradients are simultaneously created: one of “mitotic potential,” which is normally passed on to the larger daughter to facilitate its differentiation, and one of “apoptotic potential,” which is passed on to the smaller daughter and promotes its death. Although the details of these “potentials” are not yet understood, this separation within the parental cell seems crucial for ensuring that each cell reaches its proper endpoint.
Although caspases are well-known for their role in apoptosis, it is particularly noteworthy that mutations in CED-3 caspase do not only affect the ability of the small daughter cell to die. CED-3 caspase also appears to function in the division of the parental cell, suggesting a more complicated role of this molecular executioner during development.
CITATION:
Caenorhabditis elegans ced-3 Caspase Is Required for Asymmetric Divisions That Generate Cells Programmed To Die
Nikhil Mishra, Hai Wei, Barbara Conradt
GENETICS November 1, 2018 vol. 210 no. 3 983-998; https://doi.org/10.1534/genetics.118.301500