Catch up on 2015’s most popular Policy & Advocacy posts!
How labor reform might overhaul postdoc pay
A proposed rule from the U.S. Department of Labor could soon mandate that postdocs making less than $50,440 per year will be eligible for overtime pay at 1.5 times their hourly rate. Research labs are generally not prepared to track overtime hours and many do not have the additional funds available to pay postdocs above their current stipend.
Authentic ethics in synthetic biology
While the science behind the synthetic yeast genome project Sc 2.0 is cutting edge, the ethical questions surrounding it aren’t new, suggests geneticist and ethicist Debra J. H. Mathews in an interview with Sarah Bay.
Gene Drive: More research, not more regulations
A guest post from GSA Public Policy Chair Allan Spradling on regulation of gene drive research.
The State of Federal Research Funding in Genetics As Reflected By Members of the Genetics Society of America
The concern over research funding in biology in general and genetics in particular led GSA to survey our membership to learn more about the federal support of genetics at the level of individual principal investigators.
Working through the issues: Science, ethics and governance of gene drive research
A report on a National Academies of Sciences, Engineering, and Medicine meeting on Science, Ethics and Governance Considerations for Gene Drive Research.
pgEd Briefings: Increasing policymakers’ interest in genetics
Johnny Kung of the Personal Genetics Education Project at Harvard Medical School fills us in on a pgEd Congressional briefing on Capitol Hill about the latest developments and applications of emerging genetic technologies.

The Personal Genetics Education Project (pgEd) held a congressional briefing featuring Diana Bianchi, MD, Tufts University School of Medicine, Jennifer Doudna, PhD, University of California, Berkeley, and George Church, PhD, Harvard Medical School. Tuesday, November 17, 2015. Photo Credit: John Boal Photography
More questions than answers at Gene Editing Summit
Top scholars in genetics, bioengineering, ethics, and law debated the merits of human gene editing at the International Summit on Human Gene Editing; however consensus was far from achieved.
National Academies Human Gene-Editing Initiative holds public meeting
The Human Gene-Editing Initiative launched by the National Academy of Science and the National Academy of Medicine held a public meeting to provide an overview of the state of gene editing science in preparation for an international summit.
Scrutiny on fetal tissue impacts research community
The use of fetal tissue has drawn increasing public scrutiny in recent weeks as a result of secret video recordings involving the Planned Parenthood Federation of America. Getting less attention is how fetal cells can be essential research tools.
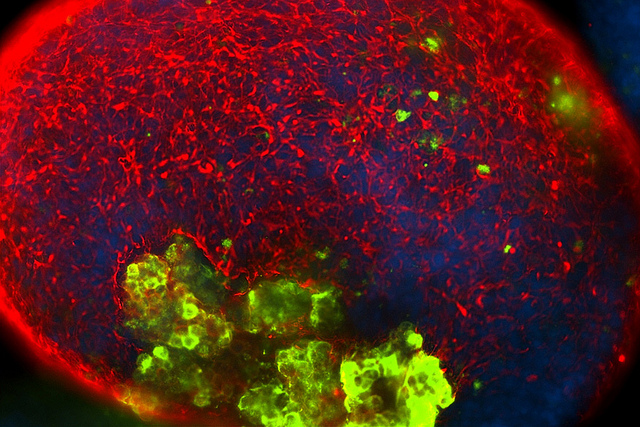
Stem cells (green) could someday be used to treat cancer, Parkinson’s disease, spinal cord injuries, Lou Gehrig’s disease, multiple sclerosis, Alzheimer’s disease and retinitis pigmentosa, among other applications. Image courtesy of Christina Tu / Sue & Bill Gross Stem Cell Research Center. CC BY-NC-ND 2.0
Agencies hold first public meeting on the GMO regulatory framework
A report on the first of three public meetings to discuss an update to the coordinated framework which serves as the regulatory guidelines for genetically engineered organisms. This meeting provided an opportunity for representatives from the primary agencies involved in GMO regulation, the Food and Drug Administration (FDA), Environmental Protection Agency (EPA), and US Department of Agriculture (USDA) to clarify their roles in the current framework.













